FAQ's
TMS is a new, breakthrough treatment for depression. Here are some common questions we are asked about TMS
Frequently Asked Questions
What Is TMS?
TMS (Transcranial Magnetic Stimulation) is a non-invasive treatment that uses magnetic pulses to stimulate specific areas of the brain involved in mood regulation. It’s often used to treat depression, especially in people who haven’t found relief from other treatments like medication or therapy.
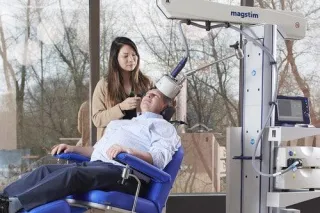
How does TMS work?
During a TMS session, a device is placed on your scalp, and it sends magnetic pulses to the brain. These pulses help activate the parts of the brain that control mood, which can improve symptoms of depression.
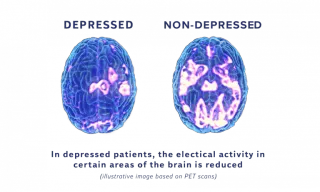
Is TMS safe?
Yes, TMS is considered safe and well-tolerated. It has been approved by the FDA for treating depression. The most common side effects are mild and include headaches or scalp discomfort, which usually go away after a few treatments.
Who can benefit from TMS?
TMS is often recommended for people with major depressive disorder (MDD) who haven’t found relief with antidepressants or therapy. It’s also being studied for other mental health conditions like anxiety and PTSD.
How long does a TMS session take?
Each TMS session usually lasts between 20 to 40 minutes. Treatment typically involves daily sessions.
Will TMS treatment hurt?
TMS is a painless procedure. You might feel a tapping sensation on your scalp during treatment, but it’s generally comfortable.
Do I need to take time off work for TMS?
Most people can continue their regular activities, including work, during TMS treatment. Sessions are short, and there’s no downtime, so you can go back to your daily routine right after each session.
When will I start to feel better?
Some people notice an improvement in their symptoms within the first few weeks of treatment, while others may take longer. Your doctor will monitor your progress and adjust the treatment if needed.
Is TMS covered by insurance?
Many insurance plans cover TMS, especially for treating depression. We recommend checking with your insurance provider to understand your specific coverage. Our team can also assist you with insurance verification.
What should I expect during my first TMS appointment?
During your first appointment, the doctor will explain the procedure, determine the right spot on your head to place the device, and set the appropriate intensity for the magnetic pulses. This session may take a little longer, but following sessions will be quicker.
Is TMS the same as electroconvulsive therapy (ECT)?
No, TMS is different from electroconvulsive therapy (ECT). While both are used to treat depression, TMS is a non-invasive procedure that uses magnetic pulses to stimulate specific areas of the brain, whereas ECT involves electrically inducing seizures under anesthesia. TMS does not require anesthesia, does not cause seizures, and has fewer side effects, making it a gentler option for treating depression.
Is TMS A Clinical Trial?
No, TMS is not a clinical trial. This is an FDA approved treatment for medication resistant depression


